Were the COVID-19 vaccines part of dual-use “Biodefence Security Covered Countermeasures,” and were they developed by the US Department of Defense prior the ‘global pandemic’?

Freddie Ponton
21st Century Wire
In analyzing the timeline of the COVID-19 pandemic response and the subsequent vaccine roll-out, the structure they have put in place is nothing short of amazing. Governments have erected an incredible shield – protected behind a legal framework which they have carefully crafted over the years, creating revolving doors and loopholes for themselves to side-step all public health regulatory measures by declaring a “Public Health Emergency” and the subsequent Emergency Use Authorization (EUA) it triggers. In hindsight, we now know that COVID-19 wasn’t a public health emergency, so why did governments go through so much trouble to protect themselves from liability, and gamble with the health and well-being of entire populations? This has never happened before in human history. Is their more to this than meet the eyes?
COVID-19 Response and Its Security Covered Countermeasures
As a result of few confirmed cases of 2019 ‘novel’ coronavirus (2019-nCoV), on 31 January, 2020, Alex M. Azar II, Secretary of Health and Human Services (HHS), pursuant to the authority vested in him under section 319 of the Public Health Service Act, determined that a public health emergency existed and had existed since 27 January, 2020 in the United States of America.
Many of us have vivid memories regarding Trump’s announcement and the subsequent launch of his ‘Operation Warp Speed’ vaccine task force in response to the coronavirus outbreak.
Coronavirus outbreak: Trump announces launch of ‘Operation Warp Speed’ vaccine task force:
As of 30 January, 2021, five of the six Operation Warp Speed (OWS) vaccine candidates had entered Phase 3 clinical trials, two of which were Moderna’s and Pfizer/BioNTech’s vaccines. We were led to believe that they had received an Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA). For vaccines that received the EUA, additional data on vaccine effectiveness were generated from further follow-up of participants in clinical trials already underway before the EUA was issued.
The questions we will be addressing in this article are essential and crucial to understand the FDA’s role during the COVID-19 pandemic response, and to establish if indeed the FDA was legally in charge of the regulatory processes including the assessment of the clinical data from the vaccine manufacturers.
Was FDA mandate adapted to ensure a strict independent review of the clinical data in the context of an Emergency Use Authorization, and was current Good Manufacturing Practice (cGMP) respected? Both questions need to be answered, as they touch upon vital measures and protocols which are expected to be in place to ensure the safety and efficacy of a new vaccine to be deployed within the American population.
FDA – The Regulatory Smoke Screen
Firstly, it’s important to identify who were the key players behind the COVID-19 pandemic response.
“Operation Warp Speed” was operating largely independently of the existing White House coronavirus task force, which we now know at some stage shifted its focus toward vaccine development.
According to the White House statement, Colonel Deborah Birx was detailed to the office of the Vice President and reported directly to Mike Pence. She was fully supported by the National Security Council (NSC) and joined the task force led by Health and Human Services (HHS) Secretary Alex Azar.
Here is a list of some of the key operatives of the Operation Warp Speed.
We could spend hours exposing the conflicts of interest many of these operatives have with Big Pharma, but for now let’s focus on Colonel Deborah Birx, President Donald Trump’s COVID-19 coordinator, and provide you with some interesting comments made by Dr Stephen M. Hahn, MD, Commissioner of U.S. Food and Drug Administration during an interview with the Reagan-Udall Foundation, and the American Medical Association (AMA) President Susan R. Bailey, MD where he outlined some aspects of the FDA’s response during the pandemic.

Everything about Colonel Deborah Birx (source)
Birx began her career with the Department of Defense, serving at Walter Reed Army Medical Center and rising to the rank of colonel while serving as the director of the U.S. Military’s HIV Research Program.
She was a strategic OWS asset, acting as what we could call “The Bridge” between the NSC statutory members, the HSS and the US DoD. Her mandate was perhaps one the of the most strategic within the federal government and by taking the time to review her career, we can better understand why she was picked by the DoD and the NSC to be the lead interface with the HHS and its various partner agencies.
Birx served as director of the Centers for Disease Control’s (CDC) Division of Global HIV/AIDS from 2005 to 2014 and brought a wealth of experience in infectious disease, immunologic, vaccine research and inter-agency coordinating capacity to address the challenges faced by both Operation Warp Speed and the National Security Council who were not shy to characterize the FDA as a ‘scientific advisory body’ rather than a regulator.
Birx became an ambassador for Global Health Diplomacy in April 2014, where she was the US government’s leader for combating AIDS globally, and she was expected to bring that expertise to the coronavirus response in 2020.
Power in the Pandemic
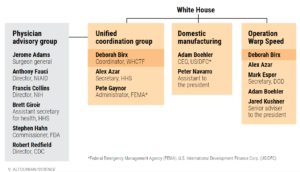
A “working organization chart” obtained by the journal Science, shows Col. Deborah Birx’s central roles in the federal COVID-19 response. She coordinated the White House Coronavirus Task Force (WHCTF) and co-chairs two of its three divisions: the Unified Coordination Group, which manages the response from the HHS and DoD, and Operation Warp Speed, whose task was to develop vaccines in cooperation with several agencies. The Physician Advisory Group had only an advisory role.
I guess this establishes the fact that FDA Commissioner Dr. Stephan Hahn was only an advisor and had no real executive power nor authorities to regulate the development and manufacturing of the various pharmaceutical products being promoted to supposedly “fight COVID”, the main ones being vaccines, or to be more accurate “medical countermeasures”.
Listed individuals co-chair the groups. (This chart was issued on 31 July 2020) source
In 2020, there was no shortage of controversies for new FDA Commissioner Dr Stephen M Hahn said the Wall Street Journal. Dr Hahn had no previous Government experience, but was somehow parachuted in around December 2019, at the helm of the FDA, where one year later he would determine that Pfizer-BioNTech’s COVID-19 mRNA ‘vaccine’ had somehow met the statutory criteria for issuance of an Emergency Use Authorization (EUA) on December 11, 2020. His decision will later reveal itself as a game changer for our safety – as Hahn completely surrendered the FDA regulatory power to the US Department of Defense (DoD), and to some extent to the Biomedical Advanced Research and Development Authority (BARDA). This was the essence of Operation Warp Speed: to bypass all the normal regulator safety rules and procedures by moving the entire effort under the aegeus of the Pentagon.
The Reagan-Udall Foundation, the American Medical Association (AMA) President Susan R. Bailey, MD and Dr. Stephen Hahn outlined the FDA’s response during the pandemic, challenges presented and information on the latest developments. (Source)
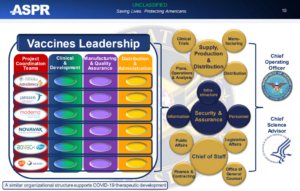
These facts are illustrated even further in this FDA document taken from a meeting presentation by the Administration for Strategic Preparedness and Response (ASPR) and their vaccines and related biological products advisory committee, October 22, 2020.
This constitutes another proof that indeed the US Department of Defense was in charge of the vaccine research & development as well as the manufacturing and logistical efforts to deploy these medical countermeasures in the US and beyond.
BARDA/ASPR/HHS COVID-19 Vaccine Development Portfolio (FDA Source):
As you can see clinical trials and their design falls under the responsibility of the US DoD, and same goes for manufacturing and distribution.
It has now become evident that the FDA was mainly in charge of “external communications”, to be provided via their public and legislative affairs council – a virtual stamp of approval necessary to assert their ‘safety and effective’ narrative for the US populace.
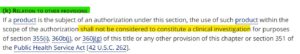
Despite all this information, we still need to establish beyond the shadow of a doubt that none of the COVID-19 clinical trials data supplied to the FDA represented an actual valid clinical investigation. That proof was provided by independent researcher Katherine Watts from Bailiwick News, a legal expert who dug deep into the legal framework governing the manufacturing of countermeasures under the US government’s Emergency Use Authorization. The 21 U.S. Code § 360bbb -3 – (k) Authorization for Medical Products for use in emergencies in section (k) “Relation to other provisions” is very precise and confirms unequivocally that the use of medical countermeasures within the scope of Emergency Use Authorization, shall not be considered to constitute a clinical investigation.
In other words, this means that none of the data which Pfizer, Moderna and the others provided to the FDA, nor any of the subjects or investigators who participated in these clinical trials – were valid within the frame of the US law.
Unfortunately, there is no other ways to look at this. According to the US Code, these clinical trials never really took place and more likely were conducted for show, and were not bona fide, and therefore were never legally established, nor could they determine the safety or efficacy of these medical countermeasures aka “vaccines.”
The 21 U.S. Code § 360bbb -3 – (k) Authorization for Medical Products for use in emergencies section (k) “Relation to other provisions”:
At this stage, and under such law, I don’t see how the FDA and the CDC can still maintain a narrative where these COVID-19 Medical Countermeasures can ever be described as ‘safe and effective’ since it appears they did not undergo any strict regulatory process, nor did they benefit from current Good Manufacturing Practice (cGMP), a fact which is well reflected in the manufacturers own data, as well as in the concerns raised by the European Medicines Agency (EMA).
In an article from Health Policy Watch, you can read the following:
“The EMA hacked documents shed light on an oft-ignored aspect of vaccine review and approval beyond safety and efficacy – that is quality-assurance of production processes as new vaccine products make the leap from clinical trials to large-scale production”. (see EMA leaked documents story here)
Dr Barbara Mintzes, Associate Professor from the University of Sydney Charles Perkins Centre and School of Pharmacy, and an expert on the interface between clinical research and regulatory decision-making, observed:
“I wonder why these issues with production quality were only made public via leaked documents that had become available on the dark web. Why were they not under open and public discussion? These types of exchanges should not be secret”.
The US VAERS data, as well as the Global Vigilance data, shows the horrific consequences following the COVID-19 ‘vaccine’ roll-out, with millions of people injured, and hundreds of thousands of people who have died following their receiving their experimental mRNA vaccine, sometimes within days and weeks after receiving the injections. You might want to bear in mind that these numbers are still very conservative, and in time hopefully we will be able to draw a more accurate picture of the damage caused by these injections.

Get Clive de Carle's Natural Health essentials of the finest quality, including vitamin & mineral supplements here.
FDA and The Public Health Emergency Medical Countermeasures Enterprise (PHEMCE)
Now that we have established that the FDA had no business, nor the authority to regulate any of the mRNA ‘vaccine’ research and development, much less the manufacturing of the COVID-19 ‘vaccine’ Countermeasures, it is time to look at the structure that has allowed this unprecedented large-scale theatrical ‘public health’ display to take place over the last 3 years.
None of this performance would have been possible unless a structure was in place to guarantee the level of discretion and secrecy required to maintain the illusion of an ongoing regulatory effort.
This structure has now been identified, and I can only strongly recommend you to watch pharmaceutical R&D and regulatory expert Sasha Latypova’s video, and also check out her Substack which covers this topic in detail. It certainly won’t fail to convince you that something went terribly wrong with the approval of these “COVID-19 Medical Countermeasures.”
The structure is called “The Public Health Emergency Medical Countermeasures Enterprise” (PHEMCE) which coordinates US federal efforts to enhance Chemical, Biological, Radiological and Nuclear (CBRN) threats and Emerging Infectious Diseases (EID) preparedness from a ‘Medical Countermeasure’ (MCM) perspective. As its website indicates:
“The PHEMCE is composed of multiple agencies across the federal government which works to optimize our preparedness for public health emergencies with respect to the creation, stockpiling, and use of medical countermeasures”.

The Public Health Emergency Medical Countermeasures Enterprise (PHEMCE)
The PHEMCE agencies constitute the perfect “SCIF” where the information stays in a vacuum and only those with a ‘need to know’ security clearance actually get to understand the bigger picture. We must also accept the possibility that many good people within these agencies had no idea what was actually going on, or how they too were duped into believing they were monitoring and regulating real clinical data – when in fact these data could never have been considered nor constituted any legitimate clinical investigation under an EUA.
Characterizing the COVID-19 Countermeasures
Back in 2020, most of us had no idea that the research and development, as well as the manufacturing and deployment of COVID-19 countermeasures, would actually fall under the control of the US Department of Defense and the National Security Council. Instead, most people believed that the COVID response efforts fell under ‘civilian’ agencies like the HSS, CDC, FDA, and NIH. When Trump announced the launch of Operation Warp Speed, the military was presented as the federal body responsible for the logistics associated with the deployment of these COVID-19 countermeasures (vaccines), and not the sole owners of the products, as indicated in their Other Transaction Agreement/Authority (OTA) signed by the vaccines companies involved in the operation.
Suffice to say that very few of us had ever heard the term “Covered Countermeasures” prior COVID-19 pandemic, hence the reason to first characterise these emergency covered countermeasures and see if indeed they rise to the level of “safe and effective,” and who controls them.
Base on what we know today, it is abundantly clear these countermeasures cannot qualify as “safe and effective” pharmaceutical products, and therefore cannot be labeled as such. The fact they are not a prophylactic, or that they do not stop infection or transmission, and that for many people they are dangerous, if not deadly, confirms they belong in an entirely different category.
So what are they?
We have reviewed many theories, and some are still under investigation, including the Bioweapon theory, the depopulation agenda theory, and even the World Economic Forum (WEF) plan to implement a digital control grid through the implementation of vaccine passports for the global population. These theories have a similar dark aura attached to them, but of course it is important to keep our feet on the ground and stick to the facts so that we can better understand the origin of these unprecedented countermeasures, and the intent that lies behind them.
First, let take a look on how the Health Secretary is categorising them before looking into the US legal code.
Covered Countermeasures:
A ‘countermeasure’ is a vaccine, a medication, a device, or other items used to prevent, diagnose, or treat a public health emergency or a security threat.
The Secretary of the Department of Health and Human Services has issued federal declarations under the Public Readiness and Emergency Preparedness Act (PREP Act) that list the countermeasures covered by the Countermeasures Injury Compensation Program (CICP).
In the declaration of the Secretary of Health on “Covered Countermeasures”, he determines the types of medical countermeasures that are covered by the CICP and declarations have been issued for medical countermeasures against the following:
- Acute Radiation Syndrome
- Anthrax
- Botulinum Toxin
- COVID-19
- Ebola
- Marburg
- Nerve Agents and Certain Insecticides (Organophophorus and/or Carbamate)
- Pandemic Influenza
- Smallpox and other orthopoxviruses (e.g., mpox)
- Zika
The characterisation of these “Covered Countermeasures” can be found also on the below Declaration Under the Public Readiness and Emergency Preparedness Act for Medical Countermeasures Against COVID–19.
Declaration Countermesures PREP ACT.
Section VI. Covered Countermeasures
As noted above, Section III of the Declaration describes the activities (referred to as ‘‘Recommended Activities’’) for which liability immunity is in effect. Section VI of the Declaration identifies the Covered Countermeasures for which the Secretary has recommended such activities. The PREP Act states that a ‘‘Covered Countermeasure’’ must be a ‘‘qualified pandemic or epidemic product,’’ or a ‘‘security countermeasure,’’ as described immediately below; or a drug, biological product or device authorized for emergency use in accordance with Sections 564, 564A, or 564B of the FD&C Act.
A ‘qualified’ pandemic or epidemic product may also be a Covered Countermeasure when it is subject to an exemption – that is, it is permitted to be used under an Investigational Drug Application or an Investigational Device Exemption, under the FD&C Act, and is ‘the object of research for possible use for diagnosis, mitigation, prevention, treatment, or cure, or to limit harm of a pandemic or epidemic or serious or life-threatening condition caused by such a drug or device.’
A security countermeasure also may be a Covered Countermeasure if it ‘may reasonably be determined to qualify for approval or licensing within 10 years after the Department’s determination that procurement of the countermeasure is appropriate.’
As the vaccines were never properly tested or taken through any of the proper legal and lawful regulatory processes designed to protect the public from risk or harm as a result of dangerous products, then the mRNA ‘vaccines’ can only rightly be classed as “prototype” counter measure products – similar to the experimental products given to, or tested on US soldiers.
Could the COVID-19 Vaccines actually be Security Covered Countermeasures?
To answer this question we needed to take into consideration the legal framework associated with these “Security Covered Countermeasures” and the subsequent requirements for their manufacturing and deployments.
In the 42 U.S. Code § 247d–6b – Strategic National Stockpile and security countermeasure procurements, in section (B) Security Countermeasure we can find more information, and I invite you to read them below:
42 U.S. Code § 247d–6b.
In the above document, the Section (4) Call for development of countermeasures: commitment for recommendation for procurement caught our attention, as it is the only Countermeasures which actually requires a Presidential approval.
In effect subsection (A) Proposal to the President indicates that if the Homeland Security Secretary and the Heath Secretary determine that a countermeasure would be appropriate (to respond to a biological threat) but is either ‘currently not developed, or unavailable for procurement as a security countermeasure, or is approved, licensed, or cleared only for alternative uses,’ the Secretaries may jointly submit to the President a proposal to etc….
In subsection (C) Presidential approval:
If the President approves a proposal under subparagraph (A), the Homeland Security Secretary and the Secretary shall make known to persons who may respond to a call for the countermeasure involved:
(i) the call for the countermeasure;
(ii) specifications for the countermeasure under subparagraph (B); and
(iii) the commitment described in subparagraph (A)(ii).
In subsection (V) Product approval:
The contract shall provide that the vendor seek approval, clearance, or licensing of the product from the Secretary; for a timetable for the development of data and other information to support such approval, clearance, or licensing; and that the Secretary may waive part or all of this contract term on request of the vendor or on the initiative of the Secretary.
And finally, you might have noticed that a special reserved fund can be used to finance the R&D, manufacturing and deployment of these “security covered countermeasures” and the definition of this special reserve fund speak for itself…
Special Reserve Fund
(2) The term “special reserve fund” means the “Biodefense Countermeasures” appropriations account, any appropriation made available pursuant to section 321j(a) of title 6, and any appropriation made available pursuant to subsection.
Our understanding so far is that these “securities covered countermeasure” were financed by a special Biodefense Countermeasures reserve fund, and are the only medical countermeasures that require the approval of the President of the United States. In other words, it requires executive authorisation.
To be sure, this is the key mechanism for this entire process.
So…
Did Trump say the quiet part out loud – admitting that he did in fact approve and authorise these military security covered countermeasures?
This is #Trump confirming he was able to get “something” approved that has proven to save a lot of lives
I was able to get the FDA to do things
This confirms these so called vaccines are actually #SecurityCountermeasures as they do require Presidential approval. Don’t be fooled pic.twitter.com/UvhSZUZ8cs
— Freddie Ponton 🇫🇷 (@LFCNewsMedia) January 19, 2023
The results seem clear at this point.
Conclusion
So we have presented our case here, and we now can comfortably say that the FDA only pretended to play the role of a regulator in charge of “safety and efficacy”, when in fact, the FDA and PHEMCE were told what to say and what to do, and what to approve – by the National Security Council and the US Department of Defense.
There was never a clinical investigation of the said vaccines, and the PHEMCE COVID cabal lured the world into believing these Biodefense Security Covered Countermeasures were somehow “safe and effective.” Clearly, they were not.
Based on this discovery, our recommendation would be that all PHEMCE senior officials must be fully interrogated in official hearings, as well as all those with statutory positions at the National Security Council, and all other department heads who were in charge of these countermeasures at the US Department of Defense.
Unfortunately, simply going after Pfizer, Moderna and the other pharmaceutical firms will likely prove to be difficult at the federal level, but not impossible if willful misconduct can be proven and established, and subsequently used as a basis for prosecution. In terms of the US justice system, our best hope may lie at the state level were an independent inquiry can be carried out by state judges and appointed oversight committees – free of federal interference. These results can then be used to leverage new and broader challenges at the federal level.
In our next report, we will cover the European response to COVID-19, and see how this new emerging information impacts the narrative that was extensively promoted on the other side of the Atlantic. We will try to identify the US DoD counterparts and helpers in Europe, and analyze the role of the European Medicines Agency (EMA), and its questionable behavior and potential responsibility in their capacity as European regulators.
All we can say for now is that: “Nothing is What It Seems….”
***
READ MORE COVID NEWS AT: 21st Century Wire Covid Files
ALSO JOIN OUR TELEGRAM CHANNEL
PLEASE HELP SUPPORT OUR INDEPENDENT MEDIA PLATFORM HERE